Extraction of metals from the ores
Extraction of metals from the ores: Overview
In this topic, we will analyse various techniques for the extraction of metals from the ores. It also discusses the dressing of the ores and the extraction of metals from crude oil with the help of examples.
Important Questions on Extraction of metals from the ores
Poling process is used for the extraction of metals like copper and tin.
In the leaching of silver, a complex is formed along with the metal precipitate.
Write the chemical equations involved in the extraction of silver by leaching(using sodium cyanide).
What is self reduction process? Name few metals which show the self-reduction process.
In the Down's process of extraction of sodium from molten sodium chloride, the reason for adding anhydride calcium chloride is:
The gas which gets collected at the anode in the electrolysis of molten is:
During the extraction of sodium from brine, water molecules undergo the following reaction:
On which electrode will this reaction take place?
Brine solution is used to obtain and by electrolyzing it. The composition of brine is:
In the electrolysis of alumina, cryolite is added to _____.
Chemical reaction does not take place when copper is added to iron sulphate solution because iron is _____ (less / more) reactive than copper.
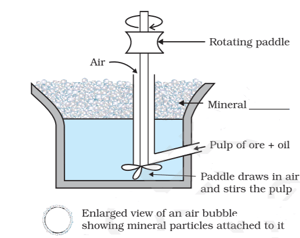
The most appropriate term for the blank in the image below is (The image below is the process of removing gangue from sulphide ores):
Out of and (ores of lead), which one is concentrated by froth floatation process preferably?
What is the role of depressants in the froth floatation process?
The process which involves smelting is
The earthy impurities associated with minerals used in metallurgy are called _____.
Which of the following reactions is an example of autoreduction?
Which of the following ores are concentrated by froth flotation?
Complete the following reaction-
+ .
Give details of the following methods for refining of metals.
Poling
Describe the Froth- Flotation process.